| Phase I clinical trial on solid tumor patients(South Korea) | |||||||||||||||||||||||||||||||
| Title | Phase I clinical trial to evaluate the safety and tolerability of oral Kominox (Kml001) as monotherapy in refractory or recurrent solid tumor patients |
||||||||||||||||||||||||||||||
| Rationale | Safety assessment of 4 weeks monotherapy
Determination of maximum tolerated dose and recommended dose of Kml001 in monotherapy |
||||||||||||||||||||||||||||||
| Dosage Regimes | 4 weeks of therapy, 1 week off cycle
|
||||||||||||||||||||||||||||||
| Results and Conclusions | Established safety for 4 weeks monotherapy
Excellent tolerability observed
Confirmed maximum tolerated dose and recommended therapeutic dose of Kml001 for Phase Ⅱ clinical trials |
||||||||||||||||||||||||||||||
| Phase I clinical trial on prostate cancer patients (Germany) | |
| Title | Phase I Clinical Trial for the Evaluation of safety and Efficacy of Kominox (Kml001) in patients with Progressive, Hormone-Resistant Prostate Cancer |
| Participants | 35 patients with advanced hormonal refractory prostate cancer (mean age = 73 years [59-84]) |
| Dosage regime | 2.5mg – 20mg, 14 days of therapy and 28 days off cycle |
| Clinical trial outcomes |
1: Determining maximum tolerated dose and toxicity profile
2: Assessment of efficacy, patient quality of life and pharmacokinetics
|
| Results and Conclusions |
Excellent tolerability: No dose limiting toxicity (DLT) observed. 4 participants experienced temporary increase in GOT/GPT by 1-2.
At least 10% of participants reported no adverse effects to Kml001 during clinical trial (such as heart rate disturbance (QT/QTc), fatigue, fever, edema, chest pain, anxiety, depression)
Disease control rate (CR+PR+SD): 66% |
| Total number of patients with and Without bone metastases | 35(100%) |
| Patients with Partial Response (PR) | 18(51%) |
| Patients with Stable Disease (SD) | 3(9%) |
| Patients with Complete Response (CR) | 2(6%) |
| Patients with Progressive Disease (PD) | 12(34%) |
| Total patients with Bone metastases | 7(100%) |
| Bone metastases Patients with Partial Response (PR) | 6(86%) |
| Bone metastases Patients with Stable Disease (SD) | 0 |
| Bone metastases Patients with Complete Response (CR) | 0 |
| Bone metastases Patients with Progressive Disease (PD) | 1(14%) |
| Phase Ⅱ Clinical Trial on Prostate Cancer (South Korea) | |
| Title | Phase 2 clinical trial of kml001 (KOMINOX) in prostate cancer patients with bone metastasis |
| No.of Participants | 13 (Mean age : 66.9, Mean weight : 70.3kg) |
| Dosage Regime | 17.5mg, 14 days therapy, 7 days off cycle |
| Clinical trial outcomes | 1: PSA response (decline of at least 50% from baseline) - an indication of no evidense of disease progression
2: PSA progression (defined by PSA increase greater than 20% from baseline) - an indication of overall patient servival rate |
| Result and Conclusions | PSA response rate of 50% observed in 4 patients / PSA progression of 30% observed in 4 patients |
| Phase Ⅱ Clinical trial on Hepatic Cancer (South Korea) | |
| Title | A comparative, open-label, multicenter phase Ⅱ clinical trial on the efficacy and safety of Kominox (Kml001) as monotherapy in patients with metastatic or locally advanced liver cancer unresponsive to chemotherapy. |
| outcomes | 4주 단독요법의 안전성 평가
Assessment of Time to Tumor Progression (TTP) and adverse reations to metermine efficacy and safety of Kominox in 4 weeks of monotherapy |
| Dosage Regime | 4주 투약 + 1주 휴약
4 weeks therapy, 1 week off cycle / 5 cycle in total, dependent on tolerability |
| Progress status(Sep 2015) | 100% patients completed the trial. / 36.0% disease control rate |
Under the relevant laws that govern the emergency use of medicines currently in clinical trials and/or not yet registered for consumer use [Article 12 of Guideline for Approval of Clinical Trial Plans for Pharmaceutical Products]
"... for intended use under the supervision of a physician and with consent of the patient, the following information must be submitted to the KFDA for approval:"
Steady Disease(SD) / Partial Response(PR) / Complete Response(CR) / Progressive Disease(PD).
| Bile Duct Cancer : 5 patients | (SD) |
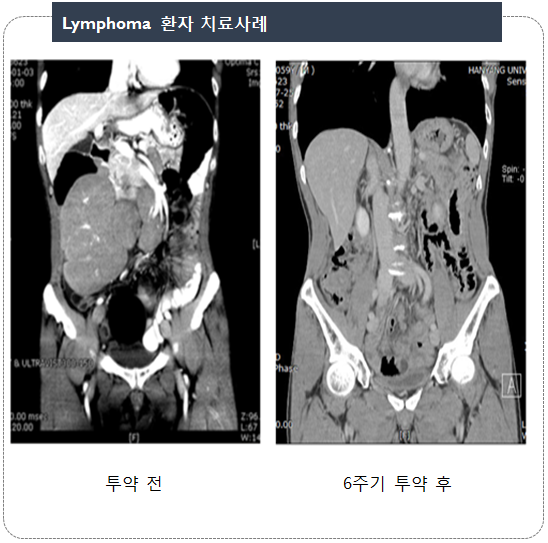
| Lymphoma : 3 patients | (PR) |
| Sarcoma : 1 patient | (SD) |
| Pancreatic cancer : 1 patient | (SD) |
| Multiple myeloma : 3 patients | (SD),(PR),(CR) |
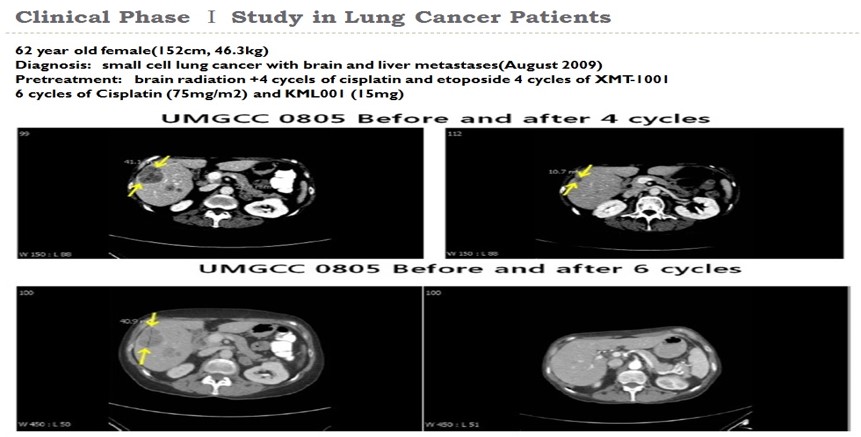
| Metastatic lung cancer : 1 patient | (CR) |